Biohaven Announces Positive Data from its Exploratory Electroencephalogram (EEG) Biomarker Study of BHV-7000, Completion of Once-Daily Formulation Development, and Plan to Initiate Phase 3 Pivotal Studies
- Examination of EEG in healthy subjects administered single doses of BHV-7000 confirmed central nervous system (CNS) activity consistent with effects observed with other antiseizure medications.
- EEG results demonstrated dose-dependent and time-dependent effects of BHV-7000 on CNS target engagement in study subjects:
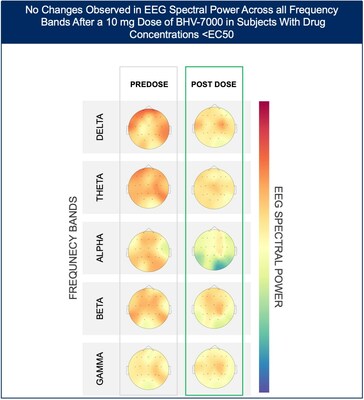
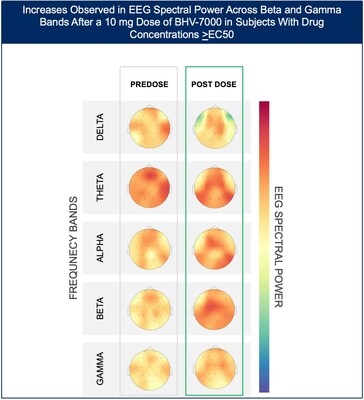
- At the lowest dose studied of 10 mg, subjects with targeted drug concentrations ≥EC50 showed a mean increase in EEG spectral power in beta and gamma bands while there were no meaningful changes in spectral power in subjects with drug concentrations <EC50.
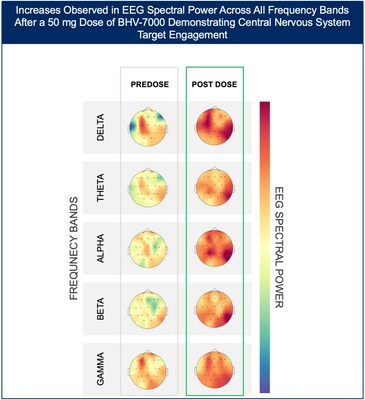
- At the highest dose studied of 50 mg, increases in EEG spectral power were observed in all frequency bands and across the entire head without distinct topographies.
- BHV-7000 has been shown to be well tolerated in Phase 1 single ascending dose (SAD) and multiple ascending dose (MAD) studies to date, with a distinct profile from other Kv7 ion channel activators and antiseizure medications.
Biohaven has also successfully completed the development of an extended-release formulation of BHV-7000 to allow for once-daily dosing to be studied in future clinical programs.- With target engagement now confirmed in the biomarker EEG study, favorable safety profile demonstrated in Phase 1 studies and development of a once-daily formulation of BHV-7000,
Biohaven plans to initiate its Phase 3 program in focal epilepsy before the end of 2023.
NEW HAVEN, Conn. and
Figure 2: Heat map depiction of topographical changes in EEG spectral power in subjects administered BHV-7000 50mg. Clear differences were observed with increased spectral power in all bands and across the entire head with no distinct topography; and, without AEs commonly associated with other ASMs including somnolence or memory impairment. Darker red color indicates a higher magnitude of spectral power.
The Phase 1 EEG study was designed to evaluate qualitative changes from baseline in EEG spectral power after administration of single doses of BHV-7000 (10, 25, or 50 mg) to healthy volunteers. EEG spectral power is a measure derived from quantitative analysis of EEG signals that assesses the amount of rhythmic activity in different frequency bands, including delta [1-3.5 Hz], theta [3.5-7.5 Hz], alpha [7.5-13 Hz], beta [13-30 Hz], and gamma [30-100 Hz]. Changes in spectral power have been used to evaluate the risk, onset and progression of seizures, assess cognitive and behavioral impairments, and characterize the effects of ASMs; and, they may also have utility in refining dose selection in clinical trials of ASMs. Spectral analysis was performed by Epilog (Ghent,
The Phase 1 EEG study showed dose-dependent and time-dependent increases in brain spectral power in healthy subjects. At the lowest dose of 10 mg (n=12), subjects with BHV-7000 concentrations ≥EC50 (based on preclinical maximal electroshock seizure (MES) models) showed mean increases in EEG spectral power in beta and gamma bands that were not observed in the group of subjects with drug concentrations < EC50 [FIGURE 1a and 1b]. These changes in beta and gamma band activity were consistent with those previously reported for other ASMs (Biondi et al. 2022). At the highest dose of 50 mg (n=11), increases in EEG spectral power were observed across all spectral bands and distributed over all cortical brain regions [FIGURE 2]. In addition to the dose-dependent observations, the time course of the increase in EEG spectral power in the 50mg dose group corresponded to the known pharmacokinetic (PK) profile of BHV-7000.
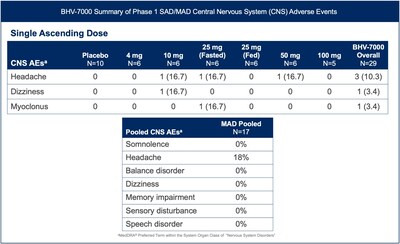
BHV-7000 was well tolerated in the exploratory EEG study and the safety profile was consistent with the previously reported safety data from the Phase 1 SAD/MAD trial completed to date in healthy volunteers [FIGURE 3]. BHV-7000 has been administered at single doses of up to 100 mg and multiple doses of up to 80 mg daily without significant CNS adverse events commonly associated with other ASMs, including a markedly lower incidence of somnolence, speech disorder and memory impairment.
Based on the results from the EEG study and preliminary safety profile in SAD/MAD trials, along with PK data from a new once-daily extended-release (ER) formulation,
About BHV-7000
BHV-7000, the lead asset from
About
Biohaven is a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies to treat a broad range of rare and common diseases. Biohaven's experienced management team brings with it a historical track record of delivering new drug approvals for products for diseases such as migraine, depression, bipolar disorder and schizophrenia.
About Epilog
Epilog is a brand of clouds of care NV, an ISO 13485:2016 and ISO 27001:2013-certified CNS marketplace. Epilog's clinical and technical EEG expertise in epileptiform disorders provides unique EEG-based insights into brain functioning. With CE-marked and FDA-cleared applications for clinicians and tailor-made solutions for clinical trials and research, Epilog is on a mission to optimize epilepsy care as part of the clouds of care CNS portfolio.
For more information, visit www.epilog.care.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of
MoDEs is a trademark of
Investor Contact:
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
mikebeyer@sambrown.com
+1 (312) 961-2502
Figure 1a: Heat map depiction of topographical changes in EEG spectral power after administration of BHV-7000 10mg in subjects with target concentrations < EC50 (based on preclinical MES models). Darker red color indicates a higher magnitude of spectral power.
Figure 1b: Heat map depiction of topographical changes in EEG spectral power after administration of BHV-7000 10mg dose in subjects with target concentrations EC50 (based on preclinical MES models). Darker red color indicates a higher magnitude of spectral power.
Figure 3: BHV-7000 Summary of Phase 1 SAD/MAD central nervous system treatment-emergent adverse events, previously presented (Biohaven Investor Presentation, August 2023).
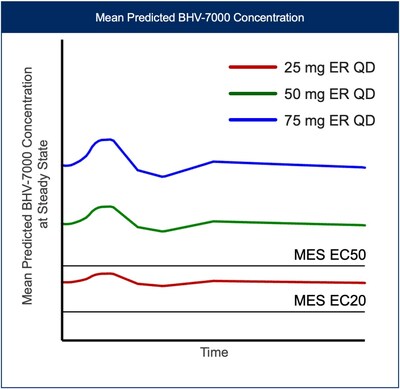
Figure 4: Predicted PK profile of BHV-7000 Extended Release (ER), mean predicted concentration vs. time profiles for 25 mg ER, 50 mg ER and 75 mg ER once-daily dosing at steady state relative to EC20 and EC50 (EC values from preclinical MES models).
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-announces-positive-data-from-its-exploratory-electroencephalogram-eeg-biomarker-study-of-bhv-7000-completion-of-once-daily-formulation-development-and-plan-to-initiate-phase-3-pivotal-studies-301917174.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-announces-positive-data-from-its-exploratory-electroencephalogram-eeg-biomarker-study-of-bhv-7000-completion-of-once-daily-formulation-development-and-plan-to-initiate-phase-3-pivotal-studies-301917174.html
SOURCE
BIOHAVEN (BHVN)
| Volume | ||
| Market Cap | ||
| 52 Week High | ||
| 52 Week Low |
Minimum 15 minutes delayed. Source: LSEG