- BHV-1300 achieved deep lowering of targeted IgG, with reductions > 60% in the lowest subcutaneous dose tested in the MAD.
- Subcutaneous BHV-1300 achieved rapid and progressive lowering of IgG within hours of each weekly dose administration, and pharmacodynamic effects were sustained relative to baseline over the four-week period. The optimized subcutaneous formulation also showed substantially less inter-patient variability in the MAD compared to the previously reported intravenously administered BHV-1300.
- BHV-1300 has been safe and well tolerated across the ongoing Phase 1 without any dose limiting toxicity to date. All AEs have been mild, with no SAEs or discontinuations related to study drug. Dose escalation continues with the optimized subcutaneous formulation to explore the full range of IgG reductions possible with BHV-1300. Additional Phase 1 data will be presented upon completion of the remaining subcutaneous cohorts in 1Q25.
- Laboratory data from the MAD confirm a differentiated safety profile compared to competitor agents as BHV-1300 has had no clinically significant reductions in albumin, liver function test abnormalities or increases in cholesterol at week 4 relative to baseline. Further enhancing the competitive safety profile, BHV-1300 was rationally designed to spare IgG3 with plasma IgG3 levels over the course of the MAD preserved through the end of study week 4 to allow for healthy immune effector functioning.
- Submitted new drug application (NDA) to US FDA for troriluzole in spinocerebellar ataxia (SCA), following completion of pre-NDA meeting in 4Q 2024. Troriluzole has Orphan Drug and Fast-Track designations and qualifies for potential Priority Review.
- Announced completion of enrollment during 4Q 2024 in the BHV-7000 pivotal 3-week, Phase 2/3 bipolar trial, several months ahead of timelines.
- Expanded and advanced the molecular degraders of extracellular proteins (MoDE) clinical program to include next-generation autoantibody specific degraders that selectively remove pathogenic antibodies while preserving healthy immune functioning, with regulatory acceptance of 3 novel drug candidate INDs and/or CTAs in 4Q24:
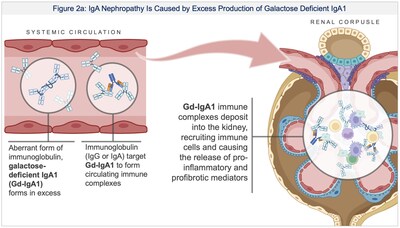
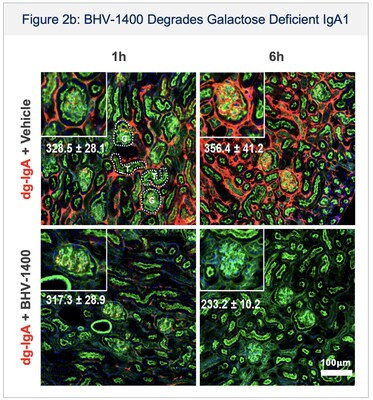
- IgA nephropathy: Initiated Phase 1 dosing with BHV-1400, a novel IgAN investigational therapy designed to selectively degrade pathogenic galactose deficient IgA1 (Gd-IgA1) while sparing normal IgA. In addition to rapid and sustained lowering of Gd-IgA1, BHV-1400 is expected to result in less potential for respiratory, mucosal or central nervous system infections compared to broader IgA lowering or immunosuppressive strategies in development by competitors.
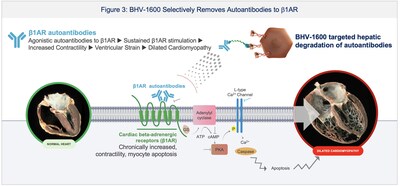
- Autoimmune cardiomyopathy: Initiated Phase 1 dosing with BHV-1600, a novel investigational therapy designed to selectively degrade b1 adrenergic receptor (b1AR) autoantibodies. Biohaven also completed an INTERACT meeting with FDA regarding BHV-1600 in 4Q 2024 and gained alignment for the study design to pursue an accelerated approval pathway in peripartum cardiomyopathy (PPCM), a rare autoimmune life-threatening disease with no approved therapy.
- IgG mediated diseases: IND opened for BHV-1310, an optimized and selective IgG1, IgG2, and IgG4 degrader in 4Q 2024 with first dosing planned for 1Q 2025.
- Entered into an agreement with Ypsomed to develop and manufacture BHV-1300 in an easy-to-use, autoinjector for self-administration. Ypsomed is a leading provider of autoinjector technology used in commercialized products for convenient patient use.
- The Ypsomed device is expected to be used across all MoDE programs through development and commercial use, derisking this aspect of the development program as well as providing seamless transitions and allowing for significant data generation on the device in advance of future NDA filings.
NEW HAVEN, Conn., Dec. 16, 2024 /PRNewswire/ -- Biohaven Ltd. (NYSE: BHVN) ("Biohaven"), a global clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of life-changing therapies to treat a broad range of rare and common diseases, today highlighted the achievement of several clinical and regulatory milestones across its proprietary Molecular Degrader of Extracellular Proteins (MoDE™) platform as well as its glutamate modulation and ion channel programs.
Subcutaneously administered BHV-1300 achieved deep lowering of targeted IgG, with reductions > 60% in the lowest subcutaneous dose tested in the ongoing multiple ascending dose (MAD) study. Subcutaneous BHV-1300 achieved progressive reduction in IgG within hours of each weekly dose administration in the MAD, and pharmacodynamic effects were sustained relative to baseline over the four-week study period. BHV-1300 has been safe and well-tolerated across the Phase 1 study. There were no clinically significant effects on albumin or liver function, and no increases in cholesterol were noted. Further enhancing the competitive safety profile and as intentionally designed, plasma IgG3 levels were preserved through the end of study week 4 to allow for healthy immune effector functioning. All AEs were mild, any drug-related AE resolved, and there were no discontinuations due to study drug related AEs. The optimized subcutaneous formulation in the MAD also showed substantially less inter-patient variability compared to previously reported intravenous BHV-1300. Escalating dose level cohorts of subcutaneous BHV-1300 are ongoing to explore the full range of IgG reductions possible with BHV-1300 for a wide range of future disease indications.
Tova Gardin MD, MPP, Biohaven's Chief Translational Officer, commented, "The results of subcutaneously delivered BHV-1300 from the first and lowest MAD dose cohort represent a monumental step forward for our MoDE platform with deep and rapid reduction in targeted IgG. Our results highlight the selectivity and precision of BHV-1300 in potentially treating IgG mediated immune diseases while the regulatory acceptance of three next generation degraders showcases the future of the MoDE technology in autoantibody disease. With speed, selectivity, and depth of lowering, BHV-1300 has the potential to transform the treatment of autoimmune disease and has paved the way for rapid innovation across the degrader platform. With the mechanistic validation of BHV-1300 and performance of the optimized subcutaneous formulation in Phase 1, we have begun the manufacturing of a convenient autoinjector that will further differentiate our approach in the clinic. The early autoinjector development to ensure a commercial ready, patient-administered device for BHV-1300 was important to derisking this aspect of our MoDE development program."
Biohaven licensed the MoDE technology from Yale University with high potential differentiation for the treatment of autoimmune diseases, including rapid degradation of targets within hours, deep reductions that can be titrated by dose level and frequency, simple-to-use patient administration, excellent tolerability without deleterious effects on albumin or cholesterol, and the flexibility to be used in conjunction with Fc-containing biologics (unlike other IgG lowering agents). Additionally, BHV-1300 is unique with its selective removal of targeted IgG (IgG1, IgG2 and IgG4 over IgG3). IgG3 plays a critical role in the immune response and in activating effector functions crucial for combating bacteria, parasites, and viruses. BHV-1300 was designed to spare IgG3 to avoid the broad immunosuppression associated with other IgG lowering approaches and to allow for an immune response to combat potential infections while delivering therapeutic actions in autoimmune disease. Results of the ongoing Phase 1 trial validate the selectivity of BHV-1300 and the adaptability of the platform to engage targets of interest with precision.

In addition to the new Phase 1 data with BHV-1300, Biohaven also announced regulatory acceptance of three INDs and/or CTAs for its next-generation MoDE molecules to target other immune mediated diseases. Two of these novel MoDEs, BHV-1400 and BHV-1600, represent the platform's first autoantibody specific degraders, sparing the body's healthy antibodies (IgG, IgA, etc.) to function normally while clearing disease-causing antibodies.
Dosing in humans has been initiated for BHV-1400, a novel IgA nephropathy (IgAN) investigational therapy designed to selectively degrade galactose deficient IgA1 (Gd-IgA1) without immunosuppression. IgAN is a rare disease affecting approximately 60,000 individuals in the United States. It is often diagnosed in the second or third decade of life, progresses over decades, and can result in kidney failure leading to the need for hemodialysis. Approved therapies broadly suppress the immune system or target the downstream consequences of immune damage without targeting the autoimmune cause of disease. For a disease which is diagnosed in young adults and treatment may be required over the lifespan, BHV-1400 is highly differentiated, engineered to clear the pathogenic nidus of disease, Gd-IgA1, and preserve the individual's immunoglobulins (IgG, IgA, IgE, IgM), immune cells, and complement system (Figure 2). Thus, BHV-1400 is expected to result in less potential for respiratory, mucosal or central nervous system infections compared to broader IgA lowering or immunosuppressive strategies in development. Additionally, IgAN clinical trials have a well-established pathway for accelerated approval.
Biohaven also initiated dosing in humans with BHV-1600 in 4Q 2024. BHV-1600 is a MoDE engineered to rapidly degrade pathogenic autoantibodies to the b1-adrenergic receptor (b1AR). BHV-1600 is the first rationally designed investigative treatment in development for peripartum cardiomyopathy (PPCM), a rare type of heart failure that occurs during pregnancy or soon after birth that has no currently approved therapy. In b1AR autoantibody-driven cardiomyopathy, autoantibodies bind b1AR and induce cardiomyocyte toxicity and heart failure (Figure 3).1 PPCM affects mothers at an incredibly vulnerable period: previously healthy, individuals with PPCM develop profound heart failure, struggling with new symptoms as their child experiences its first days and often first years of life. Without disease-specific therapies, women with PPCM can develop heart failure and may emergently require left ventricular assistive devices or heart transplant. Given PPCM is a life-threatening disease with no current treatment and the potential for BHV-1600 to rapidly degrade pathogenic b1AR autoantibodies, Biohaven completed an INTERACT meeting with the FDA in 4Q 2024 and gained alignment on a clinical development program to pursue an accelerated approval pathway for BHV-1600 in PPCM.
An IND has also been accepted for BHV-1310, an optimized and selective IgG1, IgG2, and IgG4 degrader. Dosing is expected in humans in 1Q 2025.
In addition to key updates in the MoDE clinical development program, Biohaven also announced critically important advances related to its late-stage glutamate and ion channel platforms. A new drug application (NDA) was submitted to the US FDA for troriluzole in the treatment of all genotypes of spinocerebellar ataxia (SCA), following the completion of a pre-NDA meeting in 4Q 2024. Troriluzole was previously granted Orphan Drug and Fast-Track designations by FDA, and qualifies for potential Priority Review. Biohaven recently reported positive topline pivotal results in SCA in September 2024, demonstrating that troriluzole slowed disease progression by 50-70% over the 3-year study period. SCA is a rare, life-threatening, progressively debilitating neurodegenerative disease that affects approximately 15,000 people in the US, and 24,000 in Europe and the United Kingdom. Troriluzole has been safe and well-tolerated in over 8 years of clinical trial experience. There are no FDA approved treatments for SCA and troriluzole is the first investigational agent to show disease slowing in its clinical development program.
Biohaven further announced the completion of enrollment in a pivotal BHV-7000 Phase 2/3 trial in bipolar disorder in 4Q 2024. BHV-7000 is a selective activator of Kv7 potassium channels that offers a novel and compelling mechanism of action for the treatment of bipolar disorder and an excellent tolerability at all doses evaluated in previous studies without the central nervous system adverse effects, such as somnolence and other CNS-related effects, that typically limit the use of other mood stabilizing medications. The trial completed enrollment several months ahead of anticipated timelines, reflecting the high unmet need for new treatments in bipolar disorder. The Phase 2/3 double-blind, placebo-controlled study enrolled approximately 256 patients. Patients were randomized to receive BHV-7000 75 mg once daily or placebo over a 3-week treatment period. The primary outcome measure of the study is the change from baseline to week 3 in mania symptom severity, as measured by the Young Mania Rating Scale (YMRS). Secondary objectives include response and remission rates, early onset of efficacy, depression symptom severity as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS), and safety.
Irfan Qureshi MD, Chief Medical Officer of Biohaven, commented, "Biohaven continues to propel cutting edge science into reality in the clinic. Biohaven's proprietary MoDE degrader platform is a technology that has the potential to revolutionize precision treatment of a spectrum of common and rare autoimmune diseases. Targeted removal of IgG, b1AR autoantibodies and galactose deficient IgA1 has the potential to transform the treatment of rheumatoid arthritis, myasthenia gravis, autoimmune cardiomyopathy, IgAN, and many other immune mediated disorders. While advancing new compounds in immunology and inflammation, our R&D teams have also delivered important milestones for our ion channel and glutamate modulating agents. We are proud to have completed enrollment in the pivotal bipolar study with BHV-7000, months ahead of timelines and eagerly await topline results. And, our NDA submission for spinocerebellar ataxia represents the culmination of over 8 years of innovation, research, and commitment to patients with this devastating neurodegenerative disease. We believe troriluzole is a transformative breakthrough that offers new hope to individuals and families suffering from SCA who currently have no therapeutic options to alter the course of this relentless disease." Dr. Qureshi added, "We are extremely excited about the rapid progress across the portfolio and optimistic about the opportunities we have to make a positive impact on patients' lives and create value in 2025 and beyond."
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. Biohaven is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's extensive clinical and nonclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MoDE is a trademark of Biohaven Therapeutics Ltd.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
 View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-reports-positive-phase-1-degrader-data-achieving-deep-targeted-igg-reductions-in-the-lowest-subcutaneous-dose-tested-announces-nda-submission-for-troriluzole-in-sca-and-provides-other-key-program-updates-302332472.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-reports-positive-phase-1-degrader-data-achieving-deep-targeted-igg-reductions-in-the-lowest-subcutaneous-dose-tested-announces-nda-submission-for-troriluzole-in-sca-and-provides-other-key-program-updates-302332472.html
SOURCE Biohaven Ltd.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-reports-positive-phase-1-degrader-data-achieving-deep-targeted-igg-reductions-in-the-lowest-subcutaneous-dose-tested-announces-nda-submission-for-troriluzole-in-sca-and-provides-other-key-program-updates-302332472.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-reports-positive-phase-1-degrader-data-achieving-deep-targeted-igg-reductions-in-the-lowest-subcutaneous-dose-tested-announces-nda-submission-for-troriluzole-in-sca-and-provides-other-key-program-updates-302332472.html







